Heparin-Induced Thrombocytopenia in Adult and Pediatric Patients
exp date isn't null, but text field is
Objectives
The purpose of this guideline is to provide assistance in the management of patients with suspected or documented immune mediated heparin-induced thrombocytopenia (HIT). It includes three sections: Diagnosis, Management, and Transition of Therapy.
Scope
Adult and Pediatric Patients
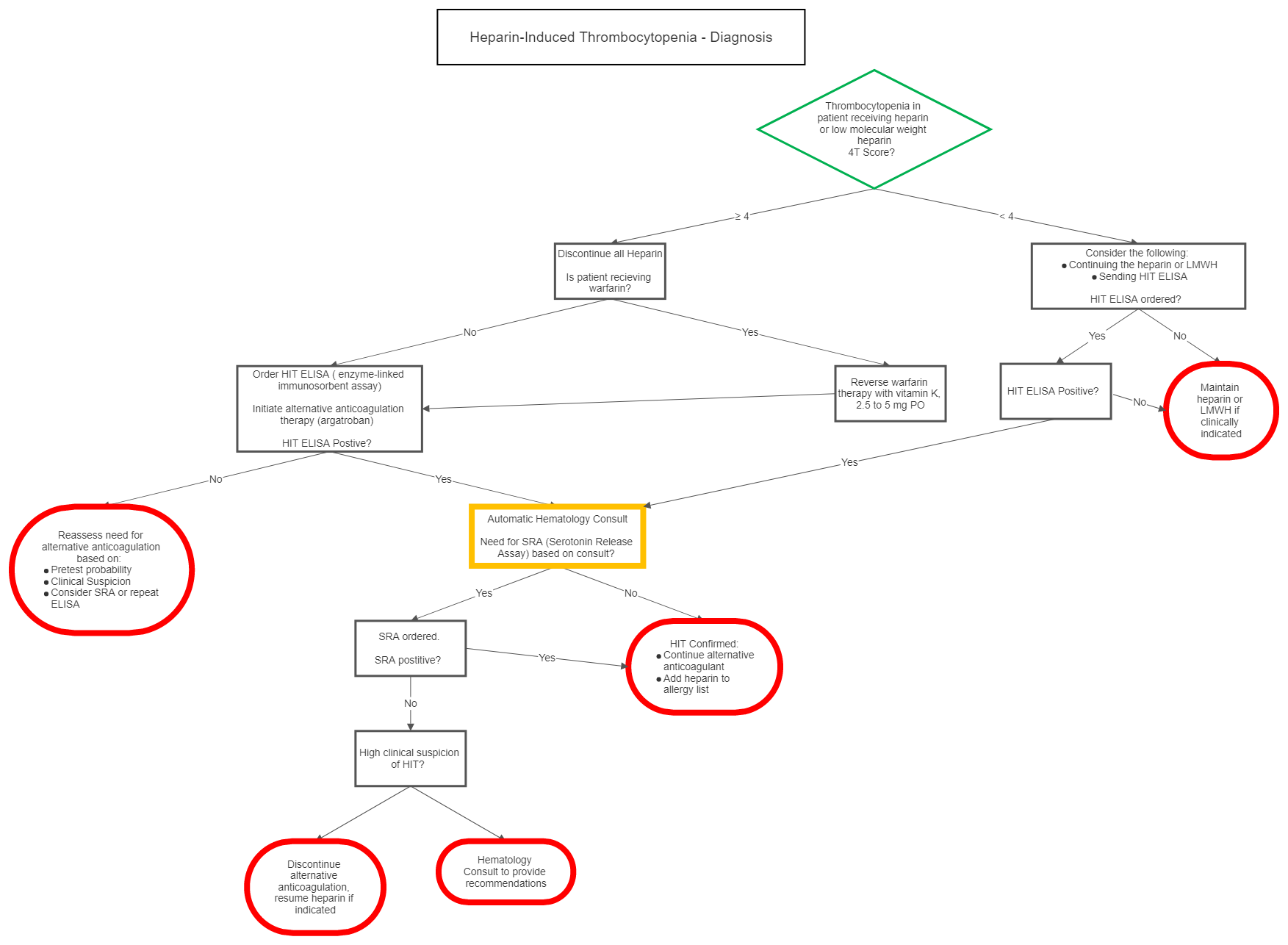
I Diagnosis
| 4T Score | 2 Points | 1 Point | 0 Points |
| Thrombocytopenia | nadir 20-100 and/or > 50% fall | nadir 10-19 or 30-50% fall | nadir < 10 or < 30% fall |
| Timing of onset of platelet fall | day 5-10 or ≤ day 1 with recent heparin (past 30 days) | > day 10, ≤ day 1 with recent heparin (past 30-100days) or timing unclear | ≤ day 4 with no recent heparin |
| Thrombosis or other sequelae | proven thrombosis, skin necrosis, or acute systemic reaction post IV bolus | progressive, recurrent or silent thrombosis; erythematous skin lesions | none |
| OTher cause of platelet fall | none evident | possible | definite |
| Probability Score: 6-8 points high, 4-5 points moderate, 0-3 points low | |||
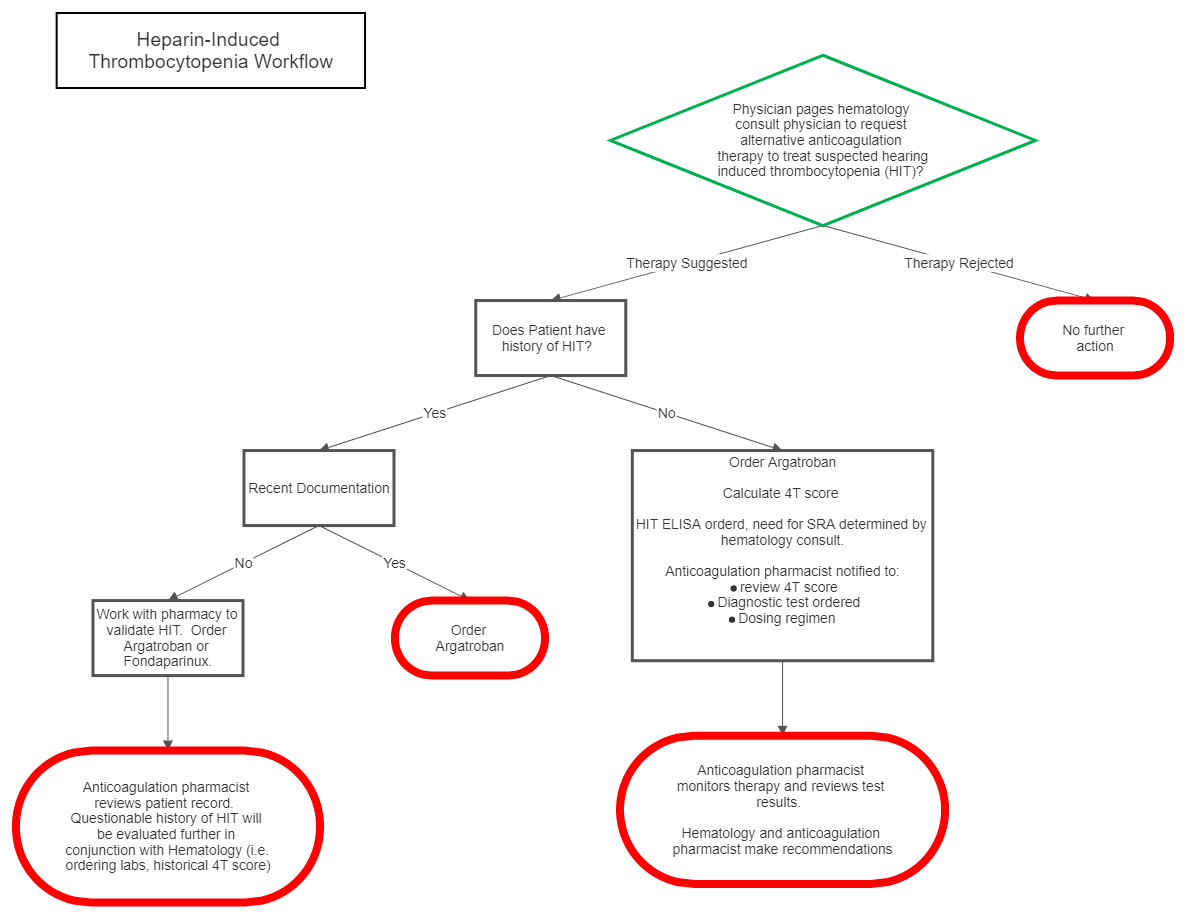
II Management
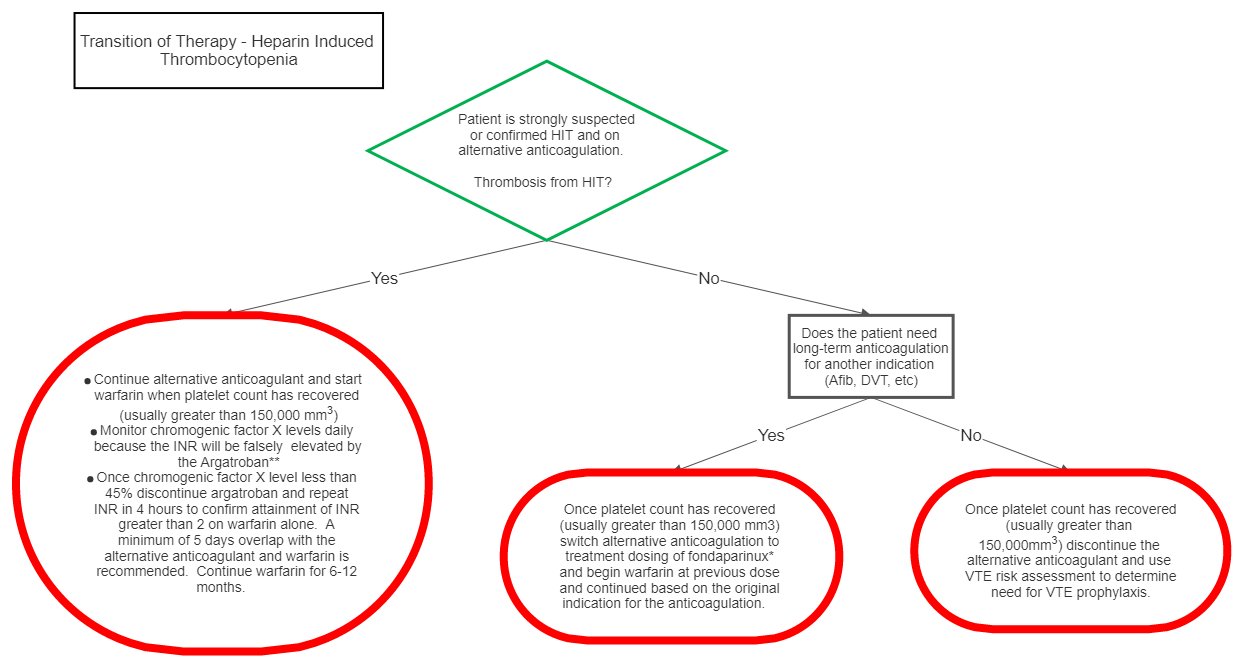
III Transition of Therapy
*Fondaparinux is chosen as the alternative anticoagulant in this scenario because of the low likelihood of the drug to cause HIT and since direct thrombin inhibitors (DTI) affect the INR, the transition of DTI’s to warfarin is difficult.
**Chromogenic Factor X levels should be used daily to monitor warfarin therapy because the INR will be falsely elevated by the DTI. Of note, the correlation between INR and chromogenic factor X is nonlinear, i.e. incremental percent reduction in chromogenic factor X does NOT represent consistent incremental increase in INR. At the lower end of the range, small reductions in chromogenic factor X levels can represent relatively larger increases in INR. Additionally, correlation between chromogenic factor X level and INR is best seen with 5 days of overlap therapy with warfarin because depletion of factor X should be at a steady state. While aggressive warfarin dosing can reach a therapeutic INR in less than 5 days, this elevation is likely representative of depletion of factor VII, however depletion of factor X will not be complete at this time resulting in a chromogenic factor X level that is not at goal. This practice can lead to supratherapeutic INR levels once the DTI is discontinued or on the contrary if the DTI is discontinued before the optimal 5 day overlap inadequate anticoagulation can occur despite therapeutic INR (secondary to depletion of factor VII but incomplete depletion of factor X and II).
Definitions:
• HIT: Heparin-induced thrombocytopenia
• SRA: Serotonin release assay
• LMWH: Low-molecular weight heparin
• EMR: Electronic medical record
• VTE: Venous thromboembolism
• INR: International normalized ratio
• DTI: Direct thrombin inhibitor