- Poor neurological outcome: Is defined as death, a persistent vegetative state or severe disability with dependence on others for activities of daily living (Cerebral Performance Category, CPC 3, 4 OR 5).
- Myoclonic status epilepticus: Generalized myoclonic status epilepticus (MSE) is defined as a prolonged state of repetitive spontaneous epileptic myoclonic activity. Myoclonus must occur at least once every 10 s for longer than 10 min or at least once a minute for longer than 30 min.1
- Hypothermic Targeted Temperature Management (HTTM): Continuous monitoring and regulation of core body temperature with target temperature between 33°C and 36°C for a minimum of 24 hours followed by gradual rewarming at a rate ≤ 0.25°C/hour followed by normothermic targeted temperature management (NTTM) for an additional 48 hours. Based on the current body of evidence, our recommended target temperature is 33°C ± 0.5°C (Bernard and HACA, 2002). However, an acceptable alternative is a target temperature of 36 ± 0.5°C (Nielson 2013). Hypothermia used to protect the brain from post-ischemic and traumatic neurological injury. The induction of mild hypothermia decreases secondary brain injury through several possible mechanisms including reduced production of free radicals, decreased transcription of cell death genes, reduced excitotoxicity, and decreased inflammatory activation.
- Normothermic Targeted Temperature Management (NTTM): Continuous monitoring and regulation of core body temperature with a target temperature of 37.0°C ± 0.5°C. The primary goal of NTTM is to prevent hyperthermia/fever, which can exacerbate brain injury caused by cardiac arrest.
- Diffuse cerebral anoxic injury on Computed Tomography (CT): Diffuse sulcal effacement and loss of grey-white differentiation in all major vascular territories of the brain (bilateral anterior and posterior circulation), indicative of diffuse cytotoxic edema. Cytotoxic cerebral edema results in loss of differentiation between cortical gray matter and medullary white matter (caused by decreased attenuatiuon in the cortical gray matter), along with effacement of sulci within the affected region (caused by displacement of cerebrospinal fluid from the sulcal space).
- Diffuse cerebral anoxic injury on magnetic resonance imaging (MRI): Diffuse, multilobar hyperintensity in all major vascular territories of the brain (bilateral anterior and posterior circulation) on Diffusion Weighted imaging (DWI) with corresponding hypointensity on the Apparent Diffusion Coefficient (ADC) map, indicative of diffuse cytotoxic edema.
- Burst suppression on electroencephalography (EEG): Greater than 50% of the EEG record with voltage <10mcV with alternating bursts. 2
Neuroprognostication in Comatose Survivors of Cardiac Arrest in Adult Intensive Care Units
exp date isn't null, but text field is
Objectives
The purpose of this document is to provide evidence-based guidelines for prognostication of neurological recovery in comatose survivors of cardiac arrest admitted to adult intensive care units.
III. POLICY STANDARDS, PROCEDURES/ACTIONS
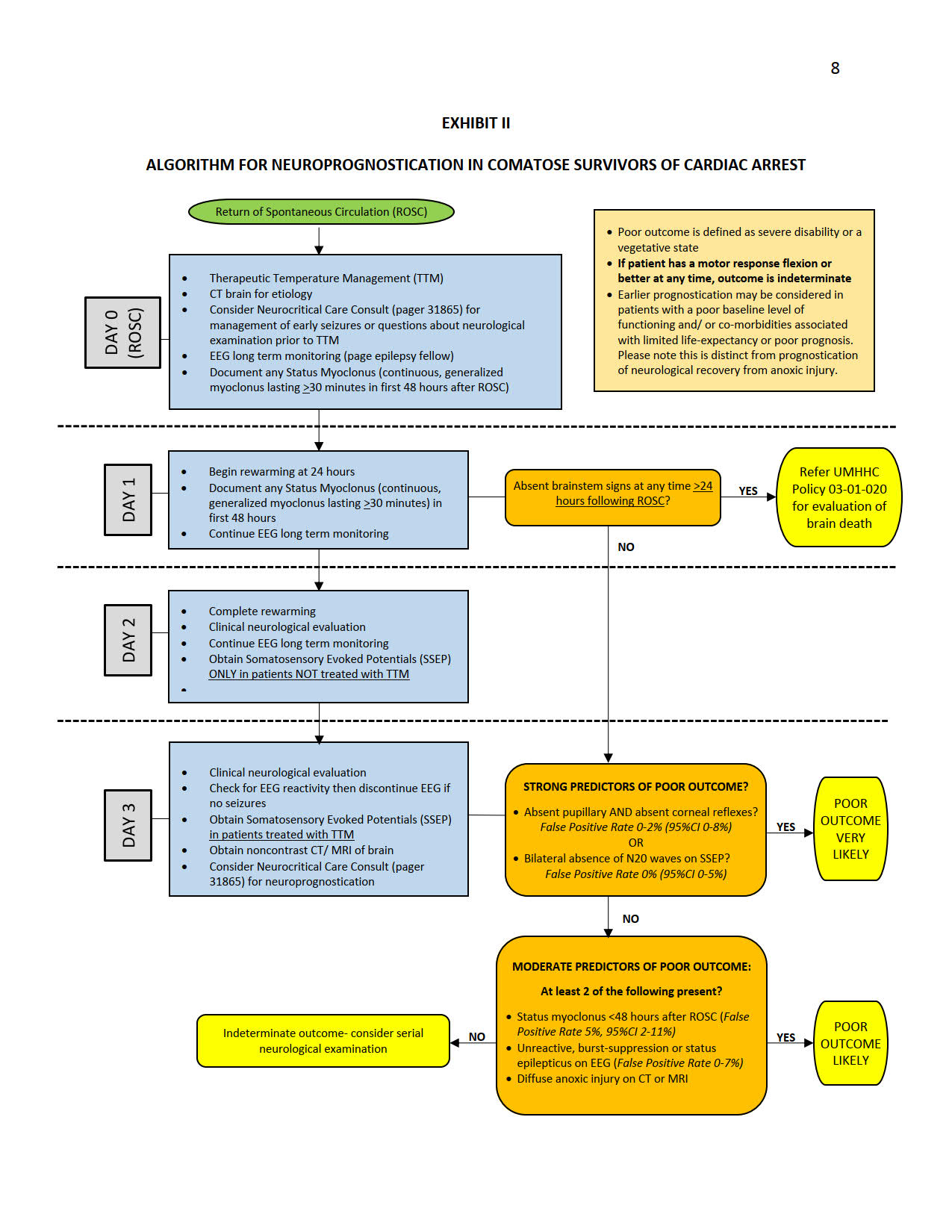
- Neuroprognostication is distinct from an assessment for brain death.
- Neurocritical Care consultation is available to assist with neuroprognostication. Early consultation may be considered for management of seizures or questions related to the neurological examination following ROSC, to select patients for use of TTM. Neuroprognostication by the neurocritical care consult team will, however, be performed at >72 hours.
- Accurate neuroprognostication is ideally performed >72 hours following Return of Spontaneous Circulation (ROSC), following completion of rewarming and at least 12 hours following cessation of all sedative and paralytic medications. Individual circumstances may justify prognostication of outcome at an earlier time point, based on a global assessment of the patient’s medical condition and co-morbidities. This may include patients with a poor baseline level of functioning and those with co-morbidities associated with limited life expectancy or poor prognosis. Please note, however, that this is distinct from prognostication of neurological recovery from anoxic injury.
- Major confounders must be excluded before an assessment for neuroprognostication is performed. These include sedation and neuromuscular blockade, persistent hypothermia, severe hypotension, hypoglycemia and other profound metabolic derangements.
- Patients who regain a motor response of flexion or better following rewarming have a reasonable possibility of good neurological recovery and are unlikely to benefit from further attempts at neuroprognostication while in the intensive care unit. In general, any sign of definitive neurological improvement over the course of evaluation should prompt careful consideration that this might indicate potentially better prognosis, and may preclude the need for further prognostic testing.
- Multimodal prognostication is recommended. Decisions to limit care based on neurologic prognosis should not be made based on the results of a single prognostication parameter.
- Continuous Electroencephalography (EEG, Long Term Monitoring), initiated as soon as possible following ROSC, should be strongly considered in all comatose survivors of cardiac arrest treated with HTTM, to monitor for potentially treatable electrographic status epilepticus and to assist with neuroprognostication.
- When assessing an EEG for prognosis, a specific verbal request should be made to the EEG fellow on call prior to the study, as that assessment requires special procedure and evaluation. Specifically, increasing levels of stimulation are required, including endotracheal suctioning, and all sedation must be absent for at least 12 hours. The report should specifically address that it is for prognostication.
- Somatosensory Evoked Potential (SSEP) testing >72 hours following ROSC should be strongly considered in all comatose survivors of cardiac arrest treated with HTTM to assist with neuroprognostication. If the patient has any reassuring clinical signs that indicate potential recovery (e.g. a reactive EEG or motor response of flexion or better), SSEP testing may be unnecessary.
- Brain imaging with noncontrast Computed Tomography (CT) should be considered >72 hours from ROSC. Magnetic Resonance Imaging (MRI) with Diffusion Weighted Imaging (DWI) may be considered when a noncontrast CT is unrevealing. The risks of performing an MRI in a critically ill patient must be taken into consideration.
- Neuroprognostication should begin with an assessment for the presence of strong predictors of poor outcome (False Positive Rate, FPR<3% with upper limit of 95% Confidence Interval, 95% CI <10%). These include the absence of pupillary and corneal reflexes, which are ideally assessed together, and the absence of bilateral N20 waves on SSEP.
- An isolated absence of either the pupillary or corneal reflex should raise concern for confounders, such as medication effect (corneal reflex) or a very sluggish pupillary reflex.
- Multiple providers should ideally confirm the absence of pupillary/ corneal reflexes.
- A poor outcome, defined as death, persistent vegetative state or severe disability, is VERY LIKELY (>95% probability, based on the available data) when pupillary and corneal reflexes are absent AND there is bilateral absence of N20 waves on SSEP.
- When only one of these strong predictors of poor outcome (absent pupillary and corneal reflexes OR bilateral absence of N20 on SSEP) are present, a poor outcome remains quite likely, however, at least one moderate predictor of poor outcome should also be present to support prognostication of poor neurological outcome.
- Strong predictors of poor outcome have relatively low prevalence and poor sensitivity. Bilateral absence of the pupillary reflex is present in about 11% of patients 72 hours following cardiac arrest and has sensitivity of about 18% for poor outcome.3 Bilateral absence of the corneal reflex is present in about 18% of patients 72 hours following cardiac arrest and has a sensitivity of about 26% for poor outcome. 3 Bilateral absence of the N20 wave is present in about 16% of patients treated with HTTM 72 hours following cardiac arrest, with sensitivity for poor outcome of about 28%.3
- When pupillary or corneal reflexes are present AND at least one N20 wave present on SSEP, the presence of two or more of the following 24 hours following the initial assessment (i.e, >96 hours) makes a poor outcome LIKELY (>80-90% probability), however, the presence of uncertainty must be acknowledged during prognostication.
- Myoclonic status epilepticus <48 hours from
- Presence of one of the following patterns on EEG- absence of reactivity to external stimuli, electrographic status epilepticus or burst suppression >72 hours following ROSC.
- Presence of diffuse loss of grey-white differentiation and sulcal effacement on head CT OR presence of a diffuse restricted-diffusion pattern on MRI-DWI.
- When none of the above criteria are present, the neurological outcome is considered indeterminate.
EXHIBITS
- EXHIBIT 1: Summary of evidence- Neuroprognostication in comatose survivors of cardiac arrest.
- EXHIBIT 2: Algorithm for neuroprognostication in comatose survivors of cardiac arrest
EXHIBIT I
NEUROPROGNOSTICATION IN COMATOSE SURVIVORS OF CARDIAC ARREST
SUMMARY OF EVIDENCE
Background:
Neuroprognostication following cardiac arrest has focused on the accurate prediction of poor outcome (defined as death, persistent vegetative state or severe disability). In 2006, the Quality Standards Subcommittee of the American Academy of Neurology (AAN) proposed an evidence-based step-wise algorithm for the prediction of poor outcome following cardiac arrest. 4 These guidelines identified three clinical predictors with a false positive rate (FPR) close to zero for the identification of patients likely to have a poor outcome (Strong evidence, Level A)- absent pupillary reflexes, absent corneal reflexes and absent or extensor motor response 72 hours following cardiac arrest. 4 The 2006 guidelines also identified the presence of myoclonic status epilepticus within the first day following circulatory arrest, absence of bilateral N20 responses on Somatosensory Evoked Potential (SSEP) testing and a serum Neuron Specific Enolase (NSE) level >33mcg/L as having “good” (Level B) evidence as predictors of poor outcome. The majority of studies that formed the basis for the 2006 guidelines were, however, conducted prior to the widespread use of Hypothermic Therapeutic Temperature Management (HTTM) for the management of comatose survivors of cardiac arrest. Since the use of Therapeutic Temperature Management (TTM), as well as the sedatives and paralytics frequently used with HTTM protocols, may have a profound impact on the clinical examination as well as, potentially, other predictors following cardiac arrest, 5 several subsequent studies, including a substudy of the TTM clinical trial, 6 have re-examined the accuracy of these predictors. 7 In 2014, the European Resuscitation Council and the European Society of Intensive Care medicine issued an advisory statement on prognostication in comatose survivors of cardiac arrest, following a review that included both studies of patients treated with HTTM as well as studies from the pre-HTTM era. 8
The purpose of this addendum is to review the role of commonly utilized clinical, electrophysiological, laboratory and imaging predictors of outcome following the use of HTTM in comatose survivors of cardiac arrest and, specifically, to identify predictors with evidence of high accuracy (FPR<3%, upper limit of 95% CI <10%) for the identification of patients likely to have a poor outcome.
The Role of Clinical Predictors:
The bilateral absence of pupillary light reflexes 72 hours following Return of Spontaneous Circulation (ROSC) appears to have a FPR of 0-2 (95% CI 0-8%) for prediction of poor outcome, but is only present in about 11% of patients 72 hours following cardiac arrest and has low sensitivity (18%).3,6-8 Bilateral absence of a corneal reflex has a slightly higher FPR of around 4% (95% CI 1-7%), possibly because the corneal reflex is more likely to be impaired by medications.
This finding has relatively low prevalence at 72 hours (18%) and low sensitivity (26%).3,6-8 Absent or extensor motor response (GCS M score <3) 72 hours following ROSC appears to have an unacceptably high FPR (~27%, 95% CI 12-48%) following use of HTTM, although some reduction in the FPR to 10-15% may be seen after 5 days. 6-8 While the presence of myoclonic status epilepticus demonstrated a near-zero FPR in studies not including patients treated with HTTM, several case reports have described excellent neurological recovery in patients with early, severe, generalized myoclonic status epilepticus treated with HTTM, with one meta-analysis suggesting an FPR of at least 5% (95% CI 2-11%).7 Myoclonic status epilepticus must be differentiated from the presence of myoclonic jerks following cardiac arrest, which have an FPR>10% in predicting poor outcome. 8
The Role of Laboratory and Clinical Electrophysiological Studies:
While a serum NSE level >33mcg/L was identified as an accurate predictor of poor outcome (predominantly in patients not treated with HTTM) in the 2006 AAN guidelines, an appropriate threshold serum value with FPR close to zero has not been identified in patients treated with HTTM. While there is some evidence to suggest that most patients treated with HTTM with an NSE level >60 mcg/L 48-72 hours following ROSC will have a poor outcome,8 other studies suggest that a higher NSE threshold (>90mcg/L) should be used to predict poor outcome. 9 Several factors may confound the use of an elevated NSE to predict outcome. 9 The bilateral absence of N20 waves on SSEP is typically less prone to confounding from HTTM and medication use than other predictors and may be the best validated diagnostic tool for the prediction of poor outcome with an FPR close to zero both during HTTM (95% CI 0-5%) and following rewarming, beyond 72 hours (95% CI 0-2%).6-8 Isolated case reports of patients treated with HTTM with good recovery despite the bilateral absence of N20 waves do, however, exist. 6-8 Potential pitfalls of SSEP include errors from artifact and the impact of the self-fulfilling prophecy. A bilateral absence of N20 waves on SSEP has relatively low prevalence at 72 hours (16%) and low sensitivity for poor outcome (28%) in patients treated with HTTM. 3 The presence of poor grey-white differentiation and diffuse sulcal effacement on a non-contrast head CT, as well the presence of a pattern of widespread restricted diffusion on Magnetic Resonance Imaging with Diffusion Weighted Imaging (MRI-DWI) at 2-5 days following ROSC has been demonstrated in several studies to predict poor outcome, however, the relatively small sample size in these studies limits the ability to reliably estimate an FPR. 8
The role of Electroencephalography (EEG) in neuroprognostication: Several EEG patterns predict poor outcome following cardiac arrest. 8
- Absence of EEG reactivity to external stimuli 48-72 hours following ROSC (FPR 0-2%, 95% CI 0-7%).8
- Electrographic status epilepticus (FPR 6%, 95% CI 2-20%).10
- Burst suppression present >72 hours following ROSC, defined as >50% of the record with voltage <10mcV with alternating bursts. 2
- Low-voltage (<20mcV) record. This finding appears to have the least specificity, with FPR up to 17%.
While the EEG may facilitate neuroprognostication, studies of EEG for neuroprognostication have had several limitations, including small sample size, lack of standardization of criteria for EEG reactivity and electrographic status epilepticus and operator dependence. The presence of low voltage alone, in particular, may be unreliable, particularly in the setting of HTTM and medication use.
Self-fulfilling prophecies: It is important to recognize that all studies of predictors of outcome following cardiac arrest, including those with near-zero FPR, are prone to confounding by the self-fulfilling prophecy. Caution must therefore be taken to not make definitive prognoses on the basis of a single predictor, including those such as SSEPs with a seemingly near-zero FPR in published studies.